Introduction:
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare neoplasm with a poor prognosis. Treatment options include CD123 directed agents (tagraxofusp) or intensive chemotherapy, followed by allogenic stem cell transplant (allo-HSCT). Sufficient evidence comparing them is lacking and there is no consensus on the best first line treatment. With the aim to compare the efficacy of the various treatment modalities in BPDCN, we performed a meta-analysis from the available literature.
Objectives:
Our primary objective was to evaluate the treatment outcomes in patients with BPDCN receiving tagraxofusp compared to various chemotherapeutic regimes, in terms of response rates, mortality, and patients proceeding to allo-HSCT.
Population:
Adult patients (age ≥ 18 years) with BPDCN who received treatment.
Outcomes:
Our primary outcome was complete response (CR), as designated by the investigators in the respective studies. Secondary outcomes included partial response (PR), no response (NR), overall survival (OS), and proportion of patients receiving allo-HSCT after therapy.
Methods:
Controlled vocabulary encompassing BPDCN and tagraxofusp or SL-401 was used to perform a systematic search in PubMed, Embase, Scopus and Cochrane from inception to June 2023. No restrictions on date, language, status, or outcomes were applied. 1291 records were identified after duplicates were removed using Mendeley. 2 reviewers independently screened titles and abstracts. We only included studies pertaining to treatment modalities in BPDCN and excluded reviews, opinions, and case reports. 36 studies (clinical trials=9, retrospective studies=20, case series=7) were identified for the final analysis. 3 studies had tagraxofusp and chemotherapy arms. They were used for Odds Ratio (OR) estimates for achieving CR using the random effects model with Mantel-Haenszel method, using RevMan. Single arm studies were utilized to determine proportions from random effects model for CR, PR, NR, mortality rate and allo-HSCT rate using OpenMeta.
Results:
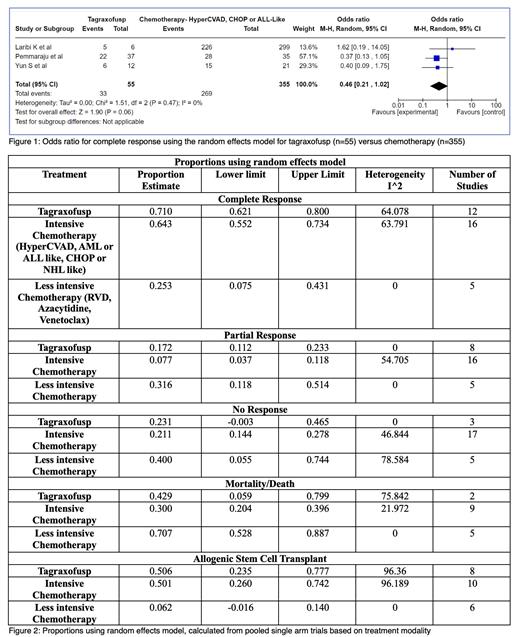
We identified 818 total patients: 36% (292/818) received tagraxofusp, 60% (494/818) received intensive chemotherapy (HyperCVAD, AML and ALL-like, and CHOP), 3% (22/818) received less intensive chemotherapy (azacytidine, venetoclax, or other mild regimes), 1% received pivekimab (10/818). The median age was 61 (range 36-80). Most patients were male and had extensive systemic disease (97%). The OR of CR for tagraxofusp versus intensive chemotherapy using the random effects model was 0.46 (confidence interval(CI)=0.21-1.02, p=0.06, I 2=0%), shown in figure 1. Proportions calculated using random effects model (figure 2) were used to determine CR (tagraxofusp 71%, intensive chemotherapy 64%, less intensive chemotherapy 25%), PR (17%, 8%, 31%), NR (23%, 21%, 40%), mortality rate (43%, 30%, 71%), and allo-HSCT rate (51%, 50%, 6%).
Conclusion:
From our analysis, the OR of attaining CR between tagraxofusp and chemotherapy was not significant and did not answer the question of which modality has a higher chance of CR. Although it is not possible to draw a direct comparison between the pooled single arm trials, the trend of proportions favored tagraxofusp for obtaining both CR (71% vs 64%) and PR (17% vs 8%). While the mortality rate was higher for tagraxofusp compared to intensive chemotherapy (43% vs 30%), the NR rate (~21%) and the allo-HSCT rate (~50%) were similar.
What is clearly seen is that less intensive therapy had a lower rate of CR (25%) and allo-HSCT (6%), and higher mortality (71%) and failure rates (PR 32%, NR 40%). This may be attributed to the baseline poor functional status of these patients, leading to the selection of less intensive therapy. We can conclude that whenever possible, either tagraxofusp or intensive chemotherapy needs to be used.
While our study does not answer the question of whether tagraxofusp or intensive chemotherapy is better as first line therapy for BPDCN, it highlights the fact that controlled data in the literature is sparse and emphasizes the need for further research in this space. Heterogeneity between the studies is a limitation of our analysis. Also, there were only a few studies directly comparing tagraxofusp and chemotherapy. Further research in a prospective, controlled setting will enable performing a more robust systematic review and meta-analysis that may help determine the best initial treatment for this rare disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal